Key Findings
UNH researchers reported the first structural model for a key enzyme, and its activating protein, that can play a role in some genetically inherited eye diseases, like retinitis pigmentosa and night blindness.
About the Co-Author

Rick Cote, Professor of Molecular, Cellular, and Biomedical Sciences
Contact information: Rick.Cote@unh.edu, 603-862-2458, Cote Lab website
This research first published in the Journal of Biological Chemistry.
Researchers: M. Irwin, R. Gupta, X. Gao, K. Cahill, F. Chu and R. Cote
Researchers at the UNH College of Life Sciences & Agriculture have reported a significant breakthrough in their paper published in the Journal of Biological Chemistry. They have identified the first structural model for a key enzyme known as PDE6, and its activating protein, that is related to some genetically inherited eye diseases such as retinitis pigmentosa and night blindness.
The team of researchers used chemical cross-linking and mass spectrometric analysis to resolve the structure of PDE6 in its nonactivated and activated states. This method allowed them to visualize the flexible regions of the individual PDE6 catalytic and inhibitory subunits that were poorly resolved in previous studies, as well as the overall molecular architecture of the activated protein complex.
At present, medical treatments for these inherited retina diseases may include gene therapy or drugs aimed at inhibiting the disease process, but they are not always successful in restoring the balance of PDE6 and preventing blindness. Scientists believe that understanding the molecular structures of these visual signaling proteins and how they interact with each other could lead to the development of new drugs to restore vision and prevent blindness.
"There has been substantial research on the biochemical pathway involving this enzyme, known as PDE6, but defining atomic-level models is important for locating PDE6 mutations in order to understand why they cause disease and how we can develop new therapeutic interventions to manage retinal diseases."
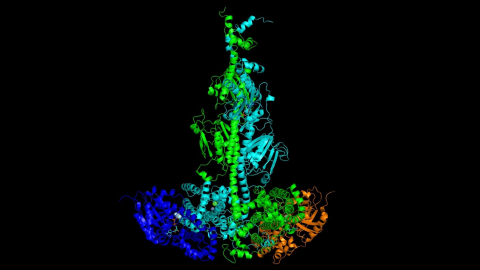
MODEL BY UNH RESEARCHERS OF THE ACTIVATED COMPLEX OF THE PDE6 MOLECULE COMPLEX (GREEN AND CYAN) WITH TWO TRANSDUCIN SUBUNITS (BLUE AND ORANGE) ASSOCIATED WITH THE PHOTORECEPTOR MEMBRANE.
Rick Cote, director of the Center of Integrated Biomedical and Bioengineering Research and principal investigator on the study, stated, "There has been substantial research on the biochemical pathway involving this enzyme, known as PDE6, but defining atomic-level models is important for locating PDE6 mutations in order to understand why they cause disease and how we can develop new therapeutic interventions to manage retinal diseases."
Michael Irwin, a doctoral student in biochemistry and lead author of the study, said, “Determining the structure of these visual signaling proteins has always been a challenge because of their complexity. Having detailed structural information about how PDE6 is activated by transducin will help us understand the molecular causes of visual disorders and blinding diseases resulting from mutations in these proteins.”
This research was funded by the National Eye Institute, the National Institute of General Medical Sciences, the National Institute of Child Health and Human Development, the National Science Foundation and the UNH Research Office. Co-authors include M. Irwin, R. Gupta, X. Gao, K. Cahill, F. Chu and R. Cote.

