Key Findings
The pathogen N. ditissima occurs more broadly than previously understood, observed in 9 of 10 sites measured and in 31 percent of trees.
Evidence also suggests N. faginata is adapting to warmer climate patterns as it disperses into more southern regions.
Beech scale insect establishment rates are lower on large, smooth beech trees and on rough-barked trees of any size, while small smooth trees are more susceptible.
About the Co-Author
Jeff Garnas, Associate Professor of Forest Ecosystem Health, Natural Resources and the Environment
Contact information: Jeff.Garnas@unh.edu, UNH Ecology and Evolution of Insects and Microbes in Forest Systems Lab
This research was published in the INSPIRED: A Publication of the New Hampshire Agricultural Experiment Station (Winter 2023)
Researchers: J. Garnas, E.W. Morrison and K. Windstein
Beech bark disease (BBD) is a widespread cankering disease of American beech (Fagus grandifolia) caused by invasive felted beech scale insects feeding on the trees and the subsequent infection by specific fungal pathogens. While the disease does kill trees, beech remains an important part of the forest ecosystem, even after many decades of infection. Despite over a century of research on this important forest disease, scientists still lack a precise understanding of the contributions of two fungal pathogens to the progression of the disease and the role of the felted beech scale in initiating BBD. The goal of this research was to understand how the fungal pathogens in the BBD system are currently distributed across the full range of this disease and how beech tree bark responds to insect and pathogen attack.
Fungal pathogens: N. faginata and N. ditissima
Beech bark disease is caused by the combined damage from the felted beech scale insect (Cryptococcus fagisuga) and then infection by two presumptively native fungal pathogens (Neonectria faginata and Neonectria ditissima). Surveys based on fruiting body (perithecia) collections have suggested that N. faginata is the dominant and more aggressive pathogen, to the point of completely excluding N. ditissima in many stands in the aftermath forest. However, differences in the timing and conditions under which either species produce fruiting bodies may bias these disease measurement methods.
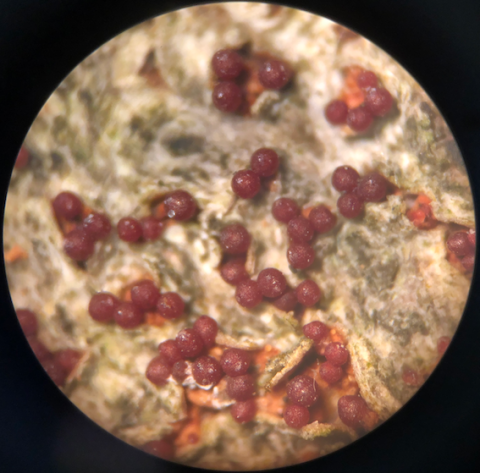
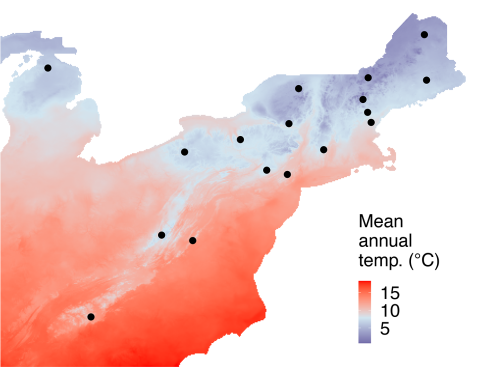
In a study of both fungal pathogens, ampliconsequencing surveys of the whole communities of fungi present in the bark of 102 beech trees across 10 sites were used to quantify occurrences of N. faginata and N. ditissima. This approach avoided potential bias inherent to fruiting body surveys. Data were also collected on perithecia (Fig. 1) from 17 sites across a range of climate conditions (Fig. 2) to examine population structure and potential adaptation to different climates. Bark samples were collected using a sterile hollow steel punch for both amplicon-sequencing and population genomics sampling. Single-spore isolations were performed from perithecia to generate a collection of fungal individuals across the range of BBD for subsequent population genomic and culture-based growth assays.
While N. faginata was generally more prevalent than N. ditissima in sites in aftermath forests, N. ditissima occurred more broadly in these forests than was previously suspected. N. ditissima was observed in 9 of the 10 sites examined and was more prevalent (two sites) or as prevalent (one site) in the three northernmost sites (Maine, Michigan and Wisconsin). The two species co-occurred in many of the trees examined, including in 27 percent of all trees (both asymptomatic and symptomatic) and 36 percent of symptomatic trees. At least one of the pathogens was detected in 55 percent of the 60 bark plugs that had no perithecia present on the periderm surface. Together, these results suggest that N. ditissima may play a greater role in disease progression than previously understood in aftermath forest stands.
Possible climate adaptation and southward spread
Joint species distribution modeling was used to examine how species occurrence related both to climate—heat accumulation during the nongrowing season of American beech—and to different measures of disease severity, including the level of crown dieback and canker abundance.
The results indicated divergent associations with climate for the two pathogens. N. faginata was positively associated with beech growing degree days, while N. ditissima displayed a negative association. Population genomic analyses also revealed potential climate adaptation in N. faginata populations.
Overall, N. faginata displayed a signal of genetic isolation by distance and little evidence of historically isolated subpopulations. The historical distribution and, in fact, the original host of this fungus prior to its novel association with beech is unknown. The lack of population substructure provides important evidence that N. faginata has dispersed in conjunction with the relatively consistent spatiotemporal spread of the beech scale, rather than having colonized beech multiple times from across an existing range. Analyses of genomic data indicate an evolutionary adaptation to warmer temperatures in N. faginata. This supports the hypothesis that there was a single-colonization event in Nova Scotia, Canada, followed by spread from cooler to warmer climates.
In total, 182 mutations were associated with non-growing season heat accumulation, with 76 of these occurring in or near one of 34 genes showing evidence of adaptation to climate. While population genomics analyses are ongoing, these initial results provide strong evidence of rapid evolution to warmer climates, which has obvious implications for the ability of N. faginata to adapt to a warming planet.
The role of the felted beech scale
The felted beech scale is a tiny, pale orange, wingless insect (Fig. 3b). Only females are known of this species, and they are legless at maturity. Their sole appendage is a straw-like feeding tube called a stylet that they plunge into the surface bark layers of their host tree, the American beech. They are most visible by the white felty wax that they produce, common on the trunks of many beech trees in this region (Fig. 3c).
The felted beech scale was introduced from eastern Europe into Nova Scotia, Canada, in 1890, spread through New Hampshire in the mid-20th century and is now present in a little over half the range of beech. Populations can become super-abundant, “white-washing” trees from top to bottom with the waxy exudates. Perhaps more importantly, however, the beech scale serves as the initiating agent of BBD, which is caused by this insect along with N. faginata and N. ditissima.
Feeding by scale insects facilitates access to the phloem—the vascular tissue in plants—for one or quite often both types of pathonegenic fungi, resulting in localized infections called cankers. BBD is responsible for elevated rates of mortality among adult beech; for the pockmarked and/or contorted form of many American beech trees that remain; and, surprisingly, for the proliferation of smaller, denser, beech-dominated stands in eastern North American forests.




Figure 3. (a) Field photos of a healthy American beech (Fagus grandifolia) tree with a rough bark phenotype that is unfavorable to scale insect establishment. (b) Invasive felted scale insect adults, one of the causal agents of beech bark disease, with white wax removed. (c) A smooth-barked, “white-washed” tree, indicating a very high scale insect establishment. (d) Bright red perithecia fungal spore-producing structures covering a highly infected beech tree.
Complex interactions among BBD agents
After well over a century of study, there are still many mysteries around this disease complex. Current research is examining how the scale insects and fungi influence one another’s populations in the “aftermath” forest, where BBD has become endemic.
One hypothesis is that the nature of the interaction between the beech scale and Neonectria fungi may have switched from one of facilitation or mutualism— during the early stages of invasion—to antagonism, and that this switch is largely mediated by bark responses of the host tree. This could explain the apparent decline in disease severity and population growth rates of BBD causal agents in long-infected forests.
To test this hypothesis, data were collected from an experiment that established small populations of insects and fungi at different densities and in different combinations on over 80 host trees and measured establishment rates for insects, lesion growth of fungal infections and host tree response to both.
Results indicate high levels of variation among individual beech trees in both scale insect establishment rates and fungal infection consistent with the expected range of genetic susceptibility to BBD, although variation in responses to fungal infection requires further study. Trees that had rough bark at the initiation of the experiment (50 percent of the sample) had significantly lower rates of scale insect establishment. Since rough bark is in large part a response to prior BBD infection, this represents a key negative feedback in the development of the disease. Interestingly, scientists found elevated scale insects on smooth bark trees, but only those in the smaller size class (10-20 cm diameter at breast height). Larger trees that maintain smooth bark are likely genetically resistant to scale attack; rates of establishment on these trees resembled that on rough barked trees of either size class.
Related published research
- Ecography: Disease ontogeny overshadows effects of climate and species interactions on population dynamics in a nonnative forest disease complex
- Mycologia: Characterization of mating type genes in heterothallic Neonectria species, with emphasis on N. coccinea, N. ditissima, and N. faginata
- Frontiers in Forests and Global Change: Pathogen and Endophyte Assemblages Co-vary With Beech Bark Disease Progression, Tree Decline, and Regional Climate
- Canadian Journal of Forest Research: Spread of beech bark disease in the eastern United States and its relationship to regional forest composition
- Journal of Ecology: Subcontinental impacts of an invasive tree disease on forest structure and dynamics
Bark lesion growth varied by fungal species, with N. faginata producing the largest lesions while eliciting the weakest host response. N. ditissima produced the smallest lesions and the largest host response. Sites that were co-inoculated with both fungi showed intermediate growth. N. ditissima responded negatively to local scale insect presence, but only on rough bark trees. N. faginata responded slightly negatively across rough and smooth bark types. At a minimum, this suggests that at this local spatial scale, insect feeding does not facilitate Neonectria growth and may in fact suppress lesion development. This offers new clues for understanding the epidemiology of this important forest disease.
Where to now?
Experimental field manipulations designed to uncover key mechanisms driving beech bark disease, and forest disease more generally, is critical to enhancing understanding and to informing management. One implication of this work is the two fungi involved in BBD clearly behave differently as they influence the disease cycle and so need to be differentiated in future studies. Luckily, this has been made much simpler by the availability of low-cost genetic screening and development of specific molecular probes for use in the BBD system. Furthermore, screening for host resistance in American beech should include consideration of the fungal pathogens and bark responses therein, which research has shown can be an important driver of BBD development over time.
This material is based on work supported by the NH Agricultural Experiment Station through joint funding from the USDA National Institute of Food and Agriculture (under Hatch award number 1023443) and the state of New Hampshire. Authored by J. Garnas, E.W. Morrison and K. Windstein.
This material is based on work supported by the NH Agricultural Experiment Station through joint funding from the USDA National Institute of Food and Agriculture (under Hatch award number 1023443) and the state of New Hampshire. Authored by J. Garnas, E.W. Morrison and K. Windstein.