Key Findings
Gastrointestinal tract microbes offer insights about the intersections of glycemic control, inflammation and appetite regulation in chronic diseases like type 2 diabetes (T2D).
The number of different types of microbes and how evenly they are distributed were found to be reduced in individuals with T2D.
About the Co-Authors
Maria Carlota Dao, Assistant Professor of Agriculture, Nutrition and Food Systems
Contact information: Carlota.Dao@unh.edu
This research was published in the INSPIRED: A Publication of the New Hampshire Agricultural Experiment Station (Summer/Fall 2023)
Researchers: B. Moser and M.C. Dao
The microbiota-gut-brain axis is thought to be a key component in the development and persistence of chronic diseases, such as type 2 diabetes (T2D). However, its relationship to inflammation and appetite regulation in the context of chronic diseases is yet to be fully understood. Further, microbiome composition is highly population-specific, as it is influenced by a multitude of factors including demographics, migration, and dietary patterns. This study aimed to better measure and understand the gut microbiome in relation to inflammation and appetite signaling in T2D in a population largely underrepresented in microbiome research.
Study Background and Design
Bhutanese refugees were the second-largest refugee group in New Hampshire in 2019. Previous research investigated the impact of SNAP-Ed nutrition curriculum and education on the health and quality of diet among the Bhutanese refugee population in New Hampshire by collecting demographic, health and dietary data on participants along with biological samples.
Using these data, this study focused on a sample size of 50 Bhutanese refugee adults in New Hampshire. Of that sample, 42% had T2D and 92% were characterized as being overweight or obese—highlighting a substantial chronic disease burden in this population.
Microbiome and Immunoassay Methods
Shallow shotgun sequences on all participants for the microbiome analysis were used, conducted with established bioinformatics pipelines for metagenomic analyses to generate measures of taxonomic abundance, richness and diversity. The inclusion of appetite-regulating hormones provided a novel component in exploring the gut microbiome and T2D. Both appetite hormones and inflammatory markers were measured using an immunoassay that harnesses chemiluminescence technology and identifies biomarker concentrations across broad concentration ranges with high specificity.
Figure 1. Family level taxonomic characterization of the gut microbiome of each study participant.
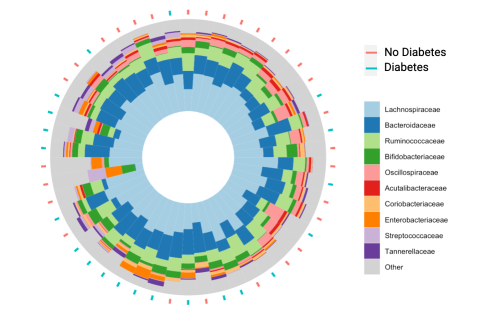
Figure 1. Family level taxonomic characterization of the gut microbiome of each study participant. Note: The red and blue dashes indicate which individuals had type 2 diabetes and those that did not. The order of arrangement represents similarities in microbial composition (unpublished results).
Microbiome-related Inflammation Associated with Glycemic Regulation
Lipopolysaccharide binding protein (LBP) is a human protein that binds with lipopolysaccharide (LPS), a component of the gram-negative bacteria cell wall, once LPS crosses the intestinal barrier and activates the immune system. Thus, LBP is considered a marker of intestinal permeability and increased levels are indicative of chronic inflammation. The occurrence of this phenomenon in the context of chronic disease has been termed metabolic endotoxemia. Researchers found that LBP concentration was positively correlated with glycemic impairment.
Microbiome Richness and Diversity Reduced in Individuals with T2D
The gut microbiome can be characterized with various methods and metrics. The relative abundance of taxonomic groups provides insight into the community structure of the gut microbiome and can be visualized by organizing individuals based on their similarities (Fig. 1). Richness of the microbiome is a measure to quantify the unique number of species present, while diversity measures encompass both the unique number of species and their distribution.

Graduate student researcher Brandy Moser pipettes plasma samples onto an assay plate in an immunoassay to quantify levels of inflammatory and satiety markers.
This study identified lower richness and diversity in those with T2D compared to those without. Although a reduction in richness and diversity has often been observed in those with obesity, findings according to T2D status have been inconsistent.
Conclusions
This study was the first characterization of the gut microbiome in Bhutanese refugee adults in New Hampshire. It aimed to explore the microbiota-gut-brain axis, specifically inflammatory and appetite regulating pathways in the context of T2D and compositional characteristics of the gut microbiome. Microbiome-related inflammation and a reduction of species richness and diversity were linked to glycemic impairment in this sample. Participant recruitment and sample collection for this study were conducted by Sherman Bigornia.
Related Published Research:
Moser, B., Milligan, M. A., Wilson, T., Dao, M. C., Flores, R., Sczytko, R., & Kanter, M. (2023). Association between inflammation, lipopolysaccharide binding protein, and gut microbiota composition in a New Hampshire Bhutanese refugee population with a high burden of type 2 diabetes. Frontiers in Nutrition, 9. https://www.frontiersin.org/articles/10.3389/fnut.2022.1059163
Moser, B., Milligan, M. A., & Dao, M. C. (2022). The microbiota-gut-brain axis: Clinical applications in obesity and type 2 diabetes. Revista de Investigacion Clinica; Organo del Hospital de Enfermedades de la Nutricion, 74(6), 302-313.
Fan, Y., & Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology, 19(1), 55-71.
Bessac, A., Jorda, M. A., Resseguier, M., Duret, C., Zoupi, L., Duneau, L., ... & Richard, D. (2018). Inflammation and gut-brain axis during type 2 diabetes: Focus on the crosstalk between intestinal immune cells and enteric nervous system. Frontiers in Neuroscience, 12, 725.


